M. Di Stefano, G. Veneto, S. Malservisi, A. Strocchi & G. R. Corazza Gastroenterology Unit, IRCCS “S. Matteo” Hospital,
University of Pavia, Pavia, Italy. Di Stefano M, Veneto G, Malservisi S, Strocchi A, Corazza GR.
Lactose malabsorption and intolerance in the elderly. Scand J Gastroenterol 2001;36:1274 –1278.
Background
Lactase activity declines with age in rats, but it is not clear whether this model is also shared by humans. Few studies have evaluated lactose intolerance and malabsorption in the elderly and no definite conclusions can be drawn. The aim of our study was therefore to verify the impact of age on lactose intolerance and malabsorption.
Methods
Eighty-four healthy subjects took part in the study. Thirty-three were <65 years, 17 were between 65 and 74 years and 34 were >74 years. All the subjects underwent a preliminary evaluation of intestinal gas production capacity and oro-cecal transit time by H2/ CH4 breath test after lactulose. After a 3-day period, an H2/CH4 breath test after lactose was performed . The occurrence of intolerance symptoms during the test and in the 24 h after the test was recorded.
Results
Breath H2 and CH4 excretion parameters at fasting and after lactulose did not differ between the three groups. Cumulative breath H2 excretion after lactose was higher in subjects >74 years than in subjects <65 years and in subjects aged 65–74 years, while no difference was found between the latter two groups. In subjects >74 years, the prevalence of lactose malabsorption was higher than in the other two groups, while no significant difference was observed between subjects <65 years and subjects aged 65–74 years. Within the malabsorber subjects, the prevalence of lactose intolerance was higher in subjects <65 years than in those aged 65–74 years and in those aged >74 years. No significant difference was found between the latter two groups. No difference was found between the three groups in terms of daily calcium intake and a significant negative correlation between symptom score and daily calcium intake was only found in the group of subjects aged <65 years.
Conclusions: As age increases, the prevalence of lactose malabsorption shows an increase while the prevalence of intolerance symptoms among malabsorbers shows a decrease. Accordingly, daily calcium intake was similar among the adults and elderly studied.
Key words:
Elderly; H2-breath test; intestinal gas production; lactose intolerance; lactose malabsorption; methane production
Human adult-type hypolactasia is widespread throughout the world due to a genetically determined decline of lactase activity inherited through an autosomal recessive gene (1). Its prevalence is highly variable, ranging from 5% in north-west Europe to almost 100% in some Asian populations (2). On clinical grounds, hypolactasia is responsible for lactose malabsorption, which may cause symptoms such as abdominal pain, bloating, flatulence and diarrhea, evoked by milk consumption (3).
In human beings, the lactase decline pattern is similar to that observed in mammals such as rats (4). However, although in rats lactase activity declines further in old age (5–7), it is not very clear whether this model is also shared by humans. The measurement of lactase activity, in fact, gave conflicting results (8, 9) probably because of the marked effect of small differences in the biopsy site (10). Only a few studies have evaluated lactose intolerance and malabsorption in the elderly by means of the hydrogen (H2) breath test (11–14), but, again, no definite conclusions could be drawn owing to differences in the methods used and in the ethnic background of the subjects.
Accordingly, the aim of this study was to verify the impact of age on lactose intolerance and malabsorption assessed by the H2 breath test. Unlike previous studies, breath H2 and methane (CH4) excretion after lactulose administration were first evaluated in all subjects to exclude the possible interference of unrelated factors such as differences in (a) H2 production capacity (15), (b) small intestine transit time (16), (c) occurrence of bacterial overgrowth (17) and (d) colonic H2 consumption (18).
SUBJECTS AND METHODS
Patients
Eighty-four healthy subjects (60 women, 24 men; age range 23–94 years) took part in the study. Thirty-three subjects were <65 years (23 women, 10 men; mean age 45 +/- 15 years). Seventeen subjects were between 65 and 74 years (12 women, 5 men; mean age 69 +/- 3 years) and 34 subjects were >74 years (25 women, 9 men; mean age 81 +/- 4 years).
Thirty-three subjects (all <65 years) were members of medical or paramedical staff of our hospital, while 51 were members of the ‘Third Age University’, an association organizing cultural and recreational events for elderly people. All were compliant and gave their informed consent to the study. Subjects with intestinal, liver, renal, chest, cardiac, metabolic or neurologic disease or who were taking antibiotics, prokinetics, laxatives or any other drug known to influence colonic flora in the month preceding the study were excluded.

Daily calcium intake was assessed in each subject by completing a dietary diary for three non-consecutive days (two non-consecutive weekdays and one weekend day) listing all the food eaten and the respective quantities, evaluated on the basis of usual portion sizes (19). The diaries were then checked by one of the authors, who was unaware of the clinical details of the subjects and analyzed on the basis of food-composition tables provided by the Italian National Institute of Nutrition (20). Moreover, each subject was asked whether milk and dairy product consumption led to appreciable abdominal symptoms.
Breath testing
In order to avoid prolonged intestinal gas production, because of the presence of non-absorbable or slowly fermentable material in the colonic lumen, the breath test was preceded by a preparation procedure based on the consumption, the evening before the test day, of a meal consisting of only rice, meat and olive oil (21). This meal was then followed by a 12-h fasting period. Breath testing started between 0830 h and 0930 h, after thorough mouth washing with 40 mL of 1% chlorhexidine solution. Smoking and physical exercise were not allowed for 1 h prior to and throughout the test.
Sampling of alveolar air was performed by means of a commercial device (Gasampler Quintron, Milwaukee, Wis., USA), which allows the first 500 mL of dead space air to be separated and discarded while the remaining 700 mL of end-alveolar air are collected in a gas-tight bag. Subjects were instructed to avoid deep inspiration and not to hyperventilate before exhalation. A gas chromatograph dedicated to the detection of H2 and CH4 in air samples was used for breath samples analysis (Model DP12, Quintron Instrument, Milwaukee, Wis., USA). The accuracy of the detector was +/- 2 ppm with a linear response range between 2 and 150 ppm of H2 and between 2 and 50 ppm of CH4.
All the subjects underwent a preliminary evaluation of intestinal gas production capacity and oro-cecal transit time by H2 breath test after the ingestion of 400 mL of an iso-osmotic solution containing 20 g of lactulose, a nonabsorbable disaccharide, which is fermented by colonic flora. Breath samples were taken at fasting and every 10 min for a 4- h period. Subjects were considered low H2 excretors if no breath sample contained an H2 concentration exceeding 20 ppm (15) and the test was considered a false-negative if an increase of breath H2 excretion greater than 20 ppm over the baseline was not evident (22). A 67-old-woman and a 75-oldwoman fulfilled these criteria and were excluded from the subsequent stages of the study. For each subject fasting, peak – that is the maximum increase over the baseline – and total excretion – evaluated by means of area under the time concentration curve calculation (23) – of H2 were recorded. Oro-cecal transit time was indicated by the presence of three sustained increments of H2 breath excretion of at least 10 ppm over the baseline (24).
Breath methane (CH4) excretion, the most important metabolic pathway of H2 consumption (25), was also measured and the prevalence of CH4 producers, i.e. subjects with mean breath CH4 excretion higher than 5 ppm, and the maximum peak, the time of appearance of the maximum peak and total breath CH4 excretion, were calculated.
After at least a 3-day period, the H2/CH4 breath test after lactose ingestion was performed. The test solution consisted of 400 mL of semi-skimmed milk, containing 20 g of lactose. Breath samples were taken at fasting and every 30 min for a 4-h period. The test was considered as indicative of the presence of lactose malabsorption when the peak of H2 breath excretion over the baseline was > 20 ppm (26, 27).
During the test, all the subjects were asked to record the occurrence of intolerance symptoms experienced during the 24-h period after the test. Bloating, abdominal pain or cramps, diarrhea and flatulence were ranked as follows: 0 = absence of the symptoms, 1 = trivial symptoms, 2 = mild symptoms, 3 = moderate symptoms, 4 = strong symptoms, 5 = severe symptoms (28). The individual scores were then added together and a mean of the individual values was given. The individual maximum score was 5.
Data Analysis
All variables were expressed as mean +/- standard deviation (s). All continuous variables showed a normal distribution with the Kolmogorov-Smirnov normality test. The unpaired t test was computed for the comparison between two groups and parametric one-way analysis of variance and Duncan’s test were used for the comparison of the means of more than Table I. Mean body mass index (BMI), triceps skin fold (TSF) and middle arm circumference (MAC) in 84 healthy subjects according to age two groups and for the multiple comparisons of the means between the pairs of groups, respectively. Differences between proportions were assessed by the chi-squared test. Pearson’s correlation coefficient was computed for the parametric estimates of the level of association between two variables. A P value <0.05 was considered significant.
Results
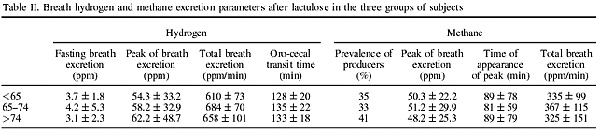
Table II indicates that subjects <65 years, between 65 and 74 years and >74 years did not significantly differ as regards a series of breath H2 and CH4 excretion parameters at fasting and after 20 g of lactulose.
Fasting breath H2 concentrations (21) and the analysis of the breath H2 excretion after lactulose administration (29) did not suggest the presence of small intestine bacterial overgrowth in any subject.
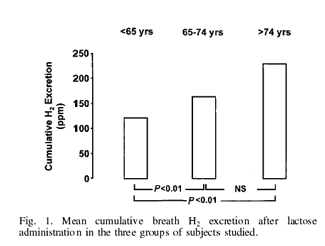
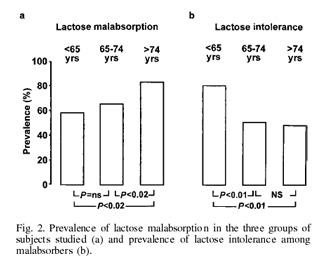
As regards lactose intolerance, all subjects but two (aged 36 and 44 years) who complained of appreciable abdominal symptoms after milk and dairy product consumption turned out to be malabsorbers at the H2 breath test. Fig. 2 also shows that within the subjects with documented lactose malabsorption the prevalence of lactose intolerance was significantly higher in subjects <65 years (80%) than in those aged 65–74 years (50%) and in those aged >74 years (48%). No significant difference was found between the latter two groups.
DISCUSSION
Our study, performed on a cohort of healthy, well-nourished subjects aged 23–94 years, shows that there is an age-dependent increase of breath H2 excretion after the ingestion of 400 mL of semi-skimmed milk. This increase can only be ascribed to lactose malabsorption, since elderly and younger adult subjects do not differ in terms of H2 production capacity by colonic flora (15), small intestine transit time (16), occurrence of bacterial overgrowth (17), and colonic H2 consumption via CH4 production (18). The observation that adults and elderly subjects show the same pattern of intestinal gas production and consumption permits us to make at least two general observations: . first, no particular interpretation of the results of H2-breath tests is necessary for elderly subjects; second, age alone does not represent a factor predisposing to alterations of intestinal gas metabolism and should not condition the clinical-diagnostic classification of elderly patients.
Our study also demonstrates that the prevalence of lactose malabsorption increases significantly after the age of 74 years. In a recent study (14) performed in a cohort of 80 healthy Caucasian women aged 40–79, the prevalence of lactose malabsorption was significantly higher in subjects aged 60–79 (50%) than in subjects aged 40–59 (15%). In this study the prevalence of lactose malabsorption in subjects aged 60–79 years was similar to that found in our healthy subjects aged 65–74 years. So, showing a further increase in subjects older than 74 years, our data complete these results, suggesting a more extended age-related decline of lactase activity during the life span. Lactose malabsorption is therefore one of the components of the broad spectrum of the aging gut and its mechanism is not easy to explain. It has been suggested that the increased enterocyte turnover shown both in aged animals (30) and humans (31) means that an increased proportion of relatively undifferentiated epithelial cells line the villi in the elderly with a consequent functional immaturity and delayed enzyme expression (5).
As far as the occurrence of symptoms after lactose administration is concerned, the prevalence of lactose intolerance among malabsorbers was significantly lower in subjects aged >65 years than in younger adults and advancing age did not determine a further decline in lactose intolerance. Our results do not agree with a previous study (14) showing no difference in prevalence of lactose intolerance between adults and elderly malabsorbers. However, such a discrepancy may be explained on the basis of a higher carbohydrate load used by those authors.
In our elderly subjects, a higher lactose malabsorption and a higher colonic H2 production after lactose administration were associated with a lower rate of lactose intolerance. Therefore, it can more reasonably be suggested that differences of viscerosensitivity between the age groups studied may be responsible for our results. Recent papers have shown that intra-esophageal balloon distension may cause a dramatic decrease in pain perception in elderly volunteers (32) and an age-related abolishment or reduction of secondary esophageal peristaltic activity (33) suggesting a possible role of abnormalities of afferent pathways and/or esophageal mechanoreceptors. It is therefore possible that similar alterations can involve the gastrointestinal tract at different levels and further studies are needed to clarify this issue.
Daily calcium intake did not differ between the three groups and a significant inverse correlation between severity of symptoms and calcium intake was evident in adult subjects only. Intolerant patients avoid milk and other dairy products, drastically reducing their daily calcium intake and exposing them to the risk of detrimental effects on bone and mineral metabolism (34). Therefore, in the light of our results, the lower prevalence of lactose intolerance may exert a protective effect in the elderly against consequent nutritional deficits. The symptom score in our malabsorbers was in any case limited and, considering differences in methods, was substantially similar to that reported in previous studies (28, 35). It can therefore be stated that malabsorbers may be allowed to follow a diet containing dairy products; a very recent paper (36) has even shown that lactose malabsorption is not an impediment to the ingestion of a dairy-rich diet supplying around 1500 mg of calcium, the dose recommended by an NIH Consensus Statement for the prevention of osteoporosis. Our results do not agree with a recent study (14) which showed a reduction of calcium intake in malabsorbers aged >70 years compared to absorbers with similar age. However, this finding was based on a small number of subjects and, moreover, data on the prevalence of lactose intolerance are also lacking. In conclusion, on the basis of our results, the ingestion of dairy products should be encouraged in the elderly, also because of the known reduced intestinal capacity to absorb calcium (37) and to adapt to a diet with low calcium concentration (38).
REFERENCES
-
Flatz G. Genetics of lactose digestion in humans. Adv Human Genet 1987;16:1–77.
-
Sahi T. Genetics and epidemiology of adult-type hypolactasia. Scand J Gastroentero l 1994;29 Suppl 202:7—20.
-
Bayless TM, Rothfeld B, Massa C, Wise L, Paige D, Bedline MS. Lactose and milk intolerance : clinical implications . N Engl J Med 1975;292:1156 –9.
-
Bu? ller HA, Kothe MJC, Goldman DA, Grubman SA, Sasak WV, Matsudaiza PT, et al. Coordinate expression of lactase-phlorizi n hydrolase mRNA and enzyme levels in rat intestine during development . J Biol Chem 1990;265:6978 –83.
-
Holt PR, Tierney AR, Kotler DP. Delayed enzyme expression: a defect of aging rat gut. Gastroenterolog y 1985;89:1026 –34.
-
Holt PR, Heller TD, Richardson AG. Food restriction retards age-related biochemical changes in rat small intestine. J Gerontol 1991;46:B89–94.
-
Lee MF, Russell RM, Montgomery RK, Krasinski SD. Total intestinal lactase and sucrase activities are reduced in aged rats. J Nutr 1997;127:1382 –7.
-
Welsh JD, Poley JR, Bhatia M, Stevenson DE. Intestinal disaccharidas e activities in relation to age, race, and mucosal damage. Gastroenterolog y 1978;75:847 –55.
-
Scand J Gastroenterol 2001 (12) Lactose and Elderly 1277
-
Wallis JL, Lipski PS, Mathers JC, James OFW, Hirst BH. Duodenal brush-border mucosal glucose transpor t and enzyme activities in aging man and effect of bacterial contamination of the small intestine. Dig Dis Sci 1993;38:403 –9.
-
Triadou N, Bataille J, Schmitz J. Longitudinal study of the human intestinal brush border membrane proteins. Distribution of the main disaccharidases and peptidases . Gastroenterology 1983;85:1326 –32.
-
Rorick MH, Scrimshaw NS. Comparative tolerance of elderly from differing ethnic backgrounds to lactose-containing and lactose-free dairy drinks: a double-blind study. J Gerontol 1979; 34:191–6.
-
Rao DR, Bello H, Warren AP, Brown GE. Prevalence of lactose maldigestion. Influence and interaction of age, race and sex. Dig Dis Sci 1994;39:1519 –24.
-
Suarez FL, Savaiano DA. Lactose digestion and tolerance in adult and elderly Asian-Americans . Am J Clin Nutr 1994;59: 1021–4.
-
Goulding A, Taylor RW, Keil D, Gold E, Lewis-Barned NJ, Williams SM. Lactose malabsorption and rate of bone loss in older women. Age Ageing 1999;28:175 –80.
-
Saltzberg DM, Levine GM, Lubar C. Impact of age, sex, race, and functional complaints on hydrogen (H2) production . Dig Dis Sci 1988;33:308–13.
-
Haboubi NY, Hudson P, Rahman Q, Lee GS, Ross A. Smallintestinal transit time in the elderly. Lancet 1988;i:933 .
-
McEvoy A, Dutton J, James OFW. Bacterial contamination of the small intestine is an important cause of occult malabsorption in the elderly. Br Med J 1983;287:789 –93.
-
Corazza GR, Benati G, Strocchi A, Malservisi S, Gasbarrini G. The possible role of breath methane measurement in detecting carbohydrate malabsorption . J Lab Clin Med 1994;124:695-700.
-
Hankin JH. Development of a diet history questionnaire for studies of older persons. Am J Clin Nutr 1989;50:1121 –7.
-
Istituto Italiano della Nutrizione. Tabelle di composizione deglialimenti. Roma, 1983.
-
Corazza GR, Strocchi A, Gasbarrini G. Fasting breath hydrogen in celiac disease. Gastroenterolog y 1987;93:53 –8.
-
Corazza GR, Strocchi A, Sorge M, Benati G, Gasbarrini G. Prevalence and consistency of low breath H2 excretion following lactulose ingestion. Possible implications for the clinical use of the H2 breath test. Dig Dis Sci 1993;38:2010 –6.
-
Kotler DP, Holt PR, Rosensweig NS. Modi. cation of the breath hydrogen test: increased sensitivity for the detection of carbohydrate malabsorption . J Lab Clin Med 1982;100:798 –805.
-
LaBrooy SJL, Male PJ, Beavis AK, Misiewicz JJ. Assessment of reproducibility of the lactulose H2 breath test as a measure of mouth to caecum transit time. Gut 1983;24:893 –6.
-
Strocchi A, Levitt MD. Factors affecting hydrogen production and consumption by human fecal flora. The critical roles of hydrogen tension and methanogenesis . J Clin Invest 1992;89: 1304–11.
-
Metz G, Jenkins DJA, Peters TJ, Newman A, Blendis LM. Breath hydrogen as a diagnostic method for hypolactasia . Lancet 1975;1:1155–7.
-
Rosado JD, Solomons NW. Sensitivity and specificity of the hydrogen breath-analysis test for detecting malabsorption of physiological doses of lactose. Clin Chem 1983;29:545–8.
-
Suarez FL, Savaiano DA, Levitt MD. A comparison of symptoms after the consumption of milk or lactose-hydrolyzed milk by people with self-reported severe lactose intolerance . N Engl J Med 1995;333:1 –4.
-
Rodhes JM, Middleton P, Jewell DP. The lactulose hydrogen breath test as a diagnostic test for small-bowel bacterial overgrowth. Scand J Gastroenterol 1979;14:333 –6.
-
Holt PR, Yeh KY, Kotler DP. Altered controls of proliferation in proximal small intestine of the senescent rat. Proc Natl Acad Sci 1988; 85:2771–5.
-
Corazza GR, Ginaldi L, Quaglione G, Ponzielli F, Vecchio L, Quaglino D, et al. Proliferating cell nuclear antigen expression is increased in small bowel epithelium in the elderly. Mech Ageing Develop 1998;104:1–9.
-
Lasch H, Castell DO, Castell JA. Evidence for diminished visceral pain with aging: studies using graded intraesophageal balloon distension. Am J Physiol 1997;272 (Gastrointestina l Liver Phisiol 35):G1–3.
-
Ren J, Shaker R, Kusano M, Podvrsan B, Metwally N, Dua KS, et al. Effect of aging on the secondary esophageal peristalsis : presbyesophagus revisited. Am J Physiol 1995;268 (Gastrointestinal Liver Physiol 31):G772–9.
-
Corazza GR, Benati G, Di Sario A, Tarozzi C, Strocchi A, Passeri M, et al. Lactose intolerance and bone mass in postmenopausal Italian women. Br J Nutr 1995;73:479–87.
-
Suarez FL, Savaiano D, Arbisi P, Levitt MD. Tolerance to the daily ingestion of two cups of milk by individuals claiming lactose intolerance . Am J Clin Nutr 1997;65:1502 –6.
-
Suarez FL, Adshead J, Furne JK, Levitt MD. Lactose maldigestion is not an impediment to the intake of 1500 mg calcium daily as dairy products . Am J Clin Nutr 1998;68:1118-22.
-
Bullamore JR, Wilkinson R, Gallagher JC, Nordin BE, Marshall DH. Effects of age on calcium absorption. Lancet 1970;2:535 –7. 38. Ireland P, Fordtran JS. Effect of dietary calcium and age on jejunal calcium absorption in humans studied by intestinal perfusion. J Clin Invest 1973;52:2672 –81.